When purchasing a new car, owners may notice that the power source used is not an ordinary lead one, but another one with the Ca/Ca marking on the body. The question arises: what is a calcium battery, how to charge and maintain it. The use of alkali metal significantly increases the basic characteristics of this battery.
Briefly about voltage and density
A typical representative of a maintenance-free calcium battery
These two parameters allow you to assess the degree of charge of a battery with liquid electrolyte. And in this particular case, we need them in order to understand whether we have charged the calcium battery correctly. Therefore, let's start with how to navigate these parameters.
Voltage
This refers to resting voltage . It can be measured correctly only after the battery has stood without any work for at least 8-10 hours (without charging or discharging). During this time, all electrochemical processes in it will gradually die out. And the tension stabilizes. Very often they forget about this nuance and measure it at random. As a result, the voltmeter shows inflated data, misleading the car enthusiast.
If you measure the rest voltage correctly, then based on the obtained indicators you can draw conclusions about the state of charge of the battery. The same applies to a dead battery. Whatever state it is in, the resting voltage will “tell” how many percent it is charged. This can be determined using a simple sign.
| Quiescent voltage (V) | Charge(%) |
| <11,90 | 0 |
| 11,95 | 10 |
| 12,00 | 20 |
| 12,05 | 30 |
| 12,15 | 40 |
| 12,20 | 50 |
| 12,30 | 60 |
| 12,40 | 70 |
| 12,50 | 80 |
| 12,60 | 90 |
| 12,70 | 100 |
| >12,71 | Incorrect measurement |
As real experience in using calcium batteries shows, the transition to a resting state often does not occur after 8-10 hours of inactivity. Sometimes you need to wait a little longer. For example, the author of this material regularly charges the calcium battery of his car with small currents for two or three days in a row. When the charge stops in the evening, in the morning the voltage at the terminals is usually more than 13 volts. This means that the state of rest has not yet arrived.
Most likely, it does not occur for so long due to the fact that the day before there was a long charge with a low current. After all, when the battery is charged regularly from the generator, the voltage is always adequate in the morning. That is, within the framework of what is listed in the plate above. From this we can conclude: the longer the calcium battery was charged, and the lower the currents, the longer it is necessary to wait for the onset of a state of rest.
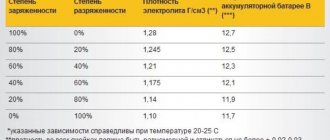
Density
This is a hydrometer - a device for measuring the density of electrolyte in a battery.
Let us immediately note that for owners of calcium batteries without plugs, this information is useless. If you have a serviceable type battery, then you definitely need to know what the density of the electrolyte is and how to use it to determine the degree of charge of the battery. It is measured with a hydrometer. You can also determine the percentage of charge using the table. Although with some reservations related to the main feature of calcium batteries. But more on that a little later. In the meantime, here's the sign.
| Density (g/cm3) | Charge(%) |
| <1,12 | 0 |
| 1,14 | 10 |
| 1,15 | 20 |
| 1,16 | 30 |
| 1,18 | 40 |
| 1,19 | 50 |
| 1,20 | 60 |
| 1,22 | 70 |
| 1,23 | 80 |
| 1,25 | 90 |
| 1,26 | 100 |
| >1,26 | Density is higher than normal |
The advantage of density compared to resting voltage is that you don’t have to wait 8-10 hours to measure it. Correct readings can be taken at any time. Even while charging the calcium battery. But let us repeat that with some reservations, which we will now begin to analyze.
or the basis of a marketing strategy
The situation today is such that, under actual operating conditions of a calcium battery, charging at 14.8 V provokes deep sulfation of the plates, and charging at 16.2 V contributes to faster melting of the active mass. In both cases, the battery capacity decreases. Each of these SEPARATE charging methods is detrimental to a calcium battery, no matter how you look at it.
However, the biggest evil that a calcium battery conceals is its intolerance to deep cycling. To put it simply, with deep discharges, the loss of capacity accelerates significantly compared to antimony batteries (the reasons are described at the beginning of the article).
Do you think the manufacturers don't know about this? They know very well, but they will never tell you about it in detail. Their goal is completely different. Their marketing strategies are based on one single advantage of the calcium battery - it is “maintenance-free” or the absence of the need to pay attention to it, in the word “set it and forget it.” But they left the showdown about the “sores” of their “brainchild” to the discretion of their consumers, since they have nothing to say. Hence the vagueness in their service recommendations. Is it any wonder now that there is an ongoing debate about the “correct” charging of a calcium battery? The answer is obvious.
Features of Ca/Ca battery
Calcium battery - structurally is a classic lead-acid battery, but with one difference. It lies in the fact that the plates in the battery are doped with calcium. That is, they are also made of lead, but covered with a microscopic coating of calcium. This was done in order to prevent the process of electrolysis of the water contained in the electrolyte.
Here and below, some concepts and processes are described in a simplified manner so that everyone can understand what we are talking about.
Electrolysis can be said to be one of the main problems of conventional batteries. It begins when the battery is more or less charged and the charge voltage exceeds 14.5 V. During the electrolysis process, water decomposes into hydrogen and oxygen, leaving the battery irrevocably. Visually, this process looks like boiling water in a kettle or pan, which is why it got its name. Although electrolysis has nothing in common with classical boiling of water except bubbling and bubbles.
This problem was solved in calcium batteries. And this had to be invented, since during the electrolysis process it is not just water that is decomposed into its constituent components. It leaves the battery, causing the electrolyte level to decrease. The electrolyte itself, at the same time, becomes more dense. The acid concentration in it increases, which is not good. Among other things, electrolysis has a detrimental effect on the condition of the lead plates of the battery. They gradually crumble and collapse, increasing the risk of a short circuit. In general, the battery life is reduced. And thanks to calcium, it increases.
But this is not all that we currently need to know about calcium batteries. The fact is that the so-called boiling in classic batteries was to some extent a useful process. Thanks to it, the electrolyte in the jars was mixed, and its density was equalized. Calcium batteries cannot boil under normal conditions. During the charging process, the more dense electrolyte falls lower, and the less dense electrolyte remains on the surface. It is because of this that, with the classical approach to charging a calcium battery, the density measured by the hydrometer cannot reach the desired level, and the indicator does not turn green.
In addition, recently there has been frequent talk about electrolyte stratification. This is the process of its stratification. We will not consider chemistry. Only three things are important to us. Firstly, due to stratification, we will never be able to correctly measure the density of the electrolyte, since it will be underestimated in the upper layers. Secondly, the increased concentration of acid in the lower part of the battery has a detrimental effect on the lead plates.
Thirdly, if suddenly such a battery is discharged in winter to more than 30%, there is every chance that there will be clean water on top and not electrolyte. It is clear that in this situation the battery will literally freeze. Sometimes it freezes so much that the battery case cannot withstand the expansion of the ice. Although this is not the only possible problem.
From all of the above, it follows that charging a calcium battery in the traditional way is not effective. Of course, he won’t get sick of it immediately. But the potential five-year resource could easily be reduced to two or three years. Actually, these are all the features you need to know about the Ca/Ca battery if your goal is to charge it correctly and fully.
Ca/Ca technology is...
View more polls later
Advantages
Calcium car batteries have the following characteristics:
- During the production process, increased strength is achieved, creating protection against vibration.
- About 90% are not serviced. Calcium reduces the electrolysis of water, causing the liquid to evaporate in small quantities.
- The plates are not afraid of overcharging, since Ca helps withstand up to 15 V.
- Thin plates allow you to increase their number in the battery, which significantly increases power.
- The alloy is considered anti-corrosion and protected from external influences.
- It has a low self-discharge rate, approximately 70% lower than that of antimony analogues.
- Durable - with proper use, service life is from 5 years.
Official instructions
How do manufacturers recommend charging a calcium battery? They usually attach short instructions to it, in which they write, without any clear explanation, what to do. One of these official instructions is now on the table, and we will take key points from it.
So, it says the following:
- A calcium battery should be charged with a current equal to 10% of its capacity.
- When the charge voltage reaches 14.4 V, the current must be halved and charged for 10 hours.
- Upon completion of the charge, it is necessary to check the density of the electrolyte and, in case of high concentration, adjust it by adding water.
- Each time after adjusting the density, the battery must be charged at a voltage of 16 V, and charged for 40 minutes.
This instruction is very good. But it has several shortcomings. The author of this instruction did not say a word about those cases when the density at the end of the charge, on the contrary, is low. He also didn’t say anything about the fact that 16 V could have an adverse effect on the car’s electronics if it occurred to you to charge the battery without disconnecting it from the on-board network. In addition, there is nothing in this instruction for those whose chargers can only be adjusted by voltage, or are generally automatic.
Therefore, let’s add a little to this instruction. Or rather, we’ll rewrite it from scratch, not forgetting about non-standard situations. We will also add a few recommendations to it that will extend the life of the calcium battery.
Technologies used
The technological process for producing calcium batteries has significant differences from the method for producing batteries doped with antimony. First of all, we had to abandon the casting method when forming lead plates, due to calcium burnout during the melting of the metal.
Calcium-doped plates are produced by stamping. This completely eliminates the possibility of metal evaporation. The principle of operation is no different from a conventional device, the plates of which are enriched with antimony, but to effectively charge the battery you will need to use a charger with a higher voltage.
Calcium battery charging algorithm
If the author of this article were assigned to write instructions for charging a calcium battery, it would look like this:
- Assess the state of charge of the battery by the resting voltage or indicator on the case.
- If the resting voltage is below 12.3 V (or the indicator is not green), charge the battery using a charger.
- Disconnect the battery from the on-board network.
- If there are plugs, remove them.
- Connect the charger, having previously set the charge current to 10% of the actual capacity, and not of the one written on the case.
- Charge until the voltage reaches 14.4 V and the charging current decreases to 0.1-0.3 A.
- If you are planning a trip soon, simply connect the battery to the car's on-board power supply.
- If you are not planning a trip, additionally charge the battery at a voltage of 16.1 V for 40 minutes.
- If the electrolyte density up to the 8th point is normal, then charging with a voltage of 16.1 V is not necessary.
- The same thing applies if the indicator on the battery case turns green.
What ultimately changed compared to the official instructions? Firstly, we eliminated the risk of damaging the car's electronics with a voltage of 16.1 V. Secondly, we made it clear that the essence of a charge with a voltage of 16.1 V is to mix electrolyte of different densities. If we are planning a trip, it will stir itself due to vibration. If not, we forcibly provoke electrolysis with high voltage, that is, we boil the battery (read later - why the battery boils). Thirdly, we made it clear that a calcium battery discharged to 60% (or below) should be charged. Otherwise, sulfation will begin.
In addition, if you carefully read the proposed algorithm for charging a calcium battery, you can understand that it is suitable for almost all types of chargers. Even for homemade ones. By the way, you might be wondering how to choose a charger.
But that's not all. Even our updated instructions cannot be called complete. It does not answer many questions that owners of calcium batteries often ask. Therefore, let’s spend a little more time and look at 10 questions regarding batteries of this type. By the way, many of the answers provided are universal. That is, they will also be useful in cases where the battery is not calcium, but some other - classic, AGM, GEL, and so on.
About desulfation of car batteries
Marking
Designations are applied to the battery to help determine their main parameters. Battery markings are divided into two categories:
- marking according to GOST (which is still in effect);
- marking according to DIN.
According to GOST standards, the marking of the 6ST-55AZ battery provides the consumer with the following data: 6 – the number of series-combined batteries in the battery, characterizing its base voltage (12V);
- ST. — purpose of the battery (starter);
- 55 – nominal type capacity in Ampere-hours;
- A – general cover, Z. – filled with electrolytic composition and fully charged.
According to the DIN standard, the marking consists of several groups of numbers:
- The first three numbers indicate the battery capacity;
- The second three numbers contain information about the design;
- The third three digits indicate the starting current value.
Marking 574 012 068 provides the following information:
- 5 - a number indicating the “order” of the capacity value;
- 74 - capacity 74 Ampere hours;
- 012 - designation of the type of housing, from which the dimensions of the housing, type of fastening, location of the terminals follow;
- 068 - starting current 680 Amps according to EN standard.
Answers to frequently asked questions
The questions discussed below are not taken out of thin air. The author of this material asked many of them at one time; friends and acquaintances were interested in others. The answers to them are not so easy to find. After all, in “prominent places” on the Internet you usually find either scant information or very different in meaning. That is, some “experts” say one thing, others, who are “experts,” contradict the first ones and put forward their own ideas. The car enthusiast can only guess - who is speaking correctly and who is wrong.
A few words should be said about where the answers to the questions presented below came from. Firstly, this is the author’s personal experience - simply using Ca/Ca batteries, as well as experiments with charging them with different chargers and in different modes. Secondly, some knowledge was gleaned from specialized books, of which at least a dozen were read. Thirdly, it is logic and common sense. As it turns out, this is important. After all, some “experts” from You Tube show this and talk about batteries, which is clear even without books with experience - it doesn’t work.
Which charger should I use to charge a calcium battery?
One of the most popular chargers for calcium batteries in 2022
If you need to avoid dancing with tambourines, then use a special charger for calcium batteries. These include those that have a 16.1 V charging mode. How to use such devices is clearly described in their operating instructions. The basic principle is based on what has already been described above.
You can charge a calcium battery with any other charger that has manual voltage adjustment. In this case, the whole process is divided into two stages. First, it charges to a terminal voltage of 14.4 V and a minimum current. Then, for about half an hour, a voltage of 16.1 V is given. In this case, the current should also be taken into account, since it can be very large if you immediately set the specified voltage.
If the charger is not special and does not provide voltage regulation, it will not be possible to charge a calcium battery stationary to 100%. However, as real experiments show, this can be achieved by mechanical stirring of the electrolyte instead of boiling with increased voltage. All that is needed for this is to install the battery on the car and drive a few kilometers on a not very smooth road. After this, as a rule, the density levels out and the indicator on the body turns green.
What voltage should I charge a calcium battery?
For a full charge, a voltage of at least 14.4 V is required. If the battery is charged stationary, then at the end the electrolyte must be forcibly mixed by electrolysis. You already know what tension to provoke it with. Under the hood, under normal conditions, there is no such problem, since everything mixes well due to vibrations. It is very useful to resort to logic and common sense here. If manufacturers know that on-board voltage on cars is 14.4-14.8 V, then they would start making batteries that cannot do without 16 V.
There are smart people on the Internet who put forward the bad idea that calcium batteries are not worth buying for a car at all. They say that there is no voltage under the hood sufficient for a 100 percent charge, which means the battery will quickly die from sulfation. This is nonsense caused by misunderstanding. Real experience confirms this. If there are no generator malfunctions in your car, the calcium battery will serve the required 5 years without any problems. Or even longer.
The author’s personal record is 11 years (shared with the previous owner of the car). This is how long the calcium battery on the car lasted, according to the production date stamped on the case. True, for the last year and a half, its capacity was at most 15 ampere-hours, and it often had to be forced with a charger. She died due to a short circuit in one of the cells.
What current should I use to charge a calcium battery?
Probably everyone knows that the charging current should be no more than 10% of the battery capacity. This is the golden rule. But it has a catch, so to speak. The fact is that the capacity of batteries constantly decreases during operation. And most car enthusiasts continue to fry them 10% of what is written on the body. This leads to the fact that the battery is not fully charged, and its resource is ultimately greatly reduced.
How does the real capacity differ from the one written on the case?
This Chinese kid can measure the actual battery capacity
Therefore, you need to understand from which capacity to take these same 10%. Of course, while the calcium battery is new, there is every chance that it has as many ampere hours as is written on the case. But after a year, this figure decreases noticeably. Even if you take care of the battery, avoiding conditions that are harmful to it. Real capacity can be measured with special devices. If you don’t want to buy them, then simply after each year of battery service, remove 10-15 ampere-hours from the original capacity on the case. Take 10% of this figure and charge it.
How long does it take to charge a calcium battery?
The charging time for a calcium battery depends on the following factors:
- actual battery capacity;
- charge current;
- degree of discharge.
The larger the capacity and degree of discharge, and the lower the current, the longer it will take to charge. On average, a completely discharged 60 ampere-hour calcium battery with an initial current of 6 A is charged in 15-20 hours. Not 10 because the battery does not “assimilate” all of its energy. Over time, this time decreases, as the battery loses capacity, and it often continues to be charged with the same six amperes. In the final stages of its life, the battery can be charged in just a couple of hours. If this is the case for you, then it will be useful for you to read how to choose a battery.
How to charge a maintenance-free calcium battery?
The only difference in this case is that it is not possible to directly measure the density of the electrolyte. Accordingly, one of the methods for assessing the state of charge of the battery is eliminated. All that remains is to navigate only by the indicator on the case, as well as by the resting voltage. In all other respects, the algorithm is the same as for serviced calcium batteries.
Is it necessary to charge with a voltage of 16 V?
No, not necessarily. If the calcium battery, by all indications, “feels” normal under the hood of the car, 16 volts are nowhere to be found. As for stationary charging, in these cases it is not at all necessary to “boil” the electrolyte with increased voltage. It will be stirred up by vibration directly on the car.
If the calcium battery is in storage, or in some kind of stationary system (for example, it accumulates energy from solar panels or a wind generator), then it is impossible to do without 16 V. Otherwise, the battery will experience sulfation of the plates and stratification of the electrolyte.
Is it possible to charge a calcium battery directly on the car?
What we mean here is whether to remove the terminals. The fact is that on many modern cars (and even not so modern ones), disconnecting the battery from the on-board network leads to certain problems. In particular, certain settings can be reset, idle speed can fluctuate, and so on. So here it is. If disconnecting the battery causes this kind of problem for you, then you can charge without removing the terminals.
However, it is still not worth giving a voltage of 16.1 V when the on-board network is connected. This often caused unprotected electronics to burn out. In particular, freelance budget gadgets - radios, recorders, alarms, navigators, etc. - often suffer from such exploitation. In such cases, it is better to hope that the electrolyte will mix during the movement.
Why doesn't the charge indicator turn green?
The indicator in a calcium battery often does not show what we would like
If you have carefully read what is written above, then you already know the answer to this question. If a calcium battery is charged without boiling at the end, the density of the electrolyte in the upper layers will be reduced. And the indicator is a kind of float that works precisely in the upper layers of the electrolyte. As a result, if the battery is not boiled, the ball does not float up and the window does not turn green. As practice shows, after a short trip on rough roads, the indicator of a battery charged without boiling turns green.
Does a new calcium battery need to be charged?
A separate article has been written on the topic of whether a new battery needs to be charged. The information presented in it applies to all types of batteries, including Ca/Ca. Go ahead. Read.
Demand creates supply
Why are calcium batteries used everywhere now? Maybe science has found a way to get rid of all the above disadvantages? Not really. The main factor here is the basic law of a market economy, which can be formulated as follows: “Demand creates supply.”
Automobile boom
Remember how many cars there were in the yards of your houses just 30 years ago? How many are there now? In any city, courtyards are literally filled with cars. There is a car boom. Previously, a car was a luxury and literally “the specks of dust were blown off”, but now? Do you think that owners of cars that “live” on the street do not sleep at night, thinking about how to service the battery on their car? Nothing like this.
Set it and forget it
People's priorities have changed a lot. The car has become a common means of transportation, and the choice of battery has shifted towards the increasingly popular “set it and forget it” principle. Whatever they say, most motorists don’t care what happens to their battery. They just don't want to deal with their service.
Here are the words of one motorist: “As practice has shown, all 3 new cars that I bought (over the last 15 years) had batteries that lasted 2 years, maximum 3” (I’ll add that this motorist is from the northern regions of Russia). Then I asked him: “Have you tried charging it?” To which he replied: “Why? It’s easier to buy a new one.” How do you like this answer? Strange, isn't it? However, there are more and more such people every day.
Competition between battery manufacturers
Now it’s clear why battery manufacturers go out of their way to offer consumers a battery that doesn’t require any attention, in a word – “set it and forget it.” And the competition among battery manufacturers is now being conducted in one single area - whose battery will last longer without any maintenance
.
Brief summary
As can be seen from the above, charging a calcium battery has its own characteristics. And all due to the fact that boiling protection is implemented in this type of battery. Under the hood it's a useful thing. But when charging from a stationary charger, there is a difficulty that has to be overcome with increased voltage. In general, as experience shows, if you charge a calcium battery regularly, promptly and correctly, take care of it and monitor it, then it will last a long time. More than 5 years. Verified by the author Auto without a service station.
A master class with a photo report on recharging a calcium battery before winter was published on the Auto without a service station website.
Recommended Chargers
Among the chargers it is worth highlighting:
- Automatic Kedr-Auto-10, costing 1,700 rubles.
- Manual Orion PW-265 and ZPU 135. Their prices are 1500 and 4000 respectively. The ZPU is a starter and has a desulfurization function, which affects its cost.
Choose your device carefully as it is not easy to charge a calcium battery correctly. A good one should include desulfurization and recovery regimes. It is desirable that it also be a starter, that is, capable of quickly replenishing the battery for a trip and subsequent full charging.